
Resonating structure of SO3 Chemistry Q&A
We are going to determine the Lewis structure for SO3 2- minus ion, also known as sulfide ion. The -2 charge you see is because it accepts two additional electrons, giving the ion a negative charge. Step-by-Step Guide to Drawing the SO3 2- Lewis Structure 1. Count Valence Electrons Begin by determining the total number […]

Draw The Lewis Dot Structure For So3 2 slidesharedocs
What is the molecular geometry of SO3 ^-2 a. draw its Lewis structure b. state its numeric code c. state its molecular geometry d. how many lone pairs of electrons are present on the central atom in the Lewis structure of sulfite ion? e.

Draw The Lewis Dot Structure For So3 2 slidesharedocs
Lewis structure of SO3 (or Sulfur trioxide) contains three double bonds between the Sulfur (S) atom and each Oxygen (O) atom. The Sulfur atom (S) is at the center and it is surrounded by 3 Oxygen atoms (O). The Sulfur atom does not have a lone pair while all the three Oxygen atoms have 2 lone pairs.

Lewis Dot Structure For So3 slidesharedocs
On the other hand for sulfurous acid, H 2SO3, we have 4 × 6 + 2 = 13 ⋅ valence electron pairs to distribute.. And thus O = .. S( − OH)2.the central sulfur is sp3 − hybridized, and the electron pairs assume a tetrahedral geometry. But molecular geometry is described in terms of ATOMS not electron pairs.and so the geometry around.

Resonance Structures So2 So3 No2 So3 2 Nitrite
Hello Guys!The sulfite ion comprises one Sulfur Atom and three Oxygen atoms. The ion has a negative charge as it accepts two additional electrons. The video.

Chemistry Worksheets, Chemistry Notes, Lewis, Dots, Activities, Development, Structures, Stitches
The Lewis structure of sulfite [SO3]2- ion is made up of a sulfur (S) atom and three oxygen (O) atoms. The sulfur (S) is present at the center of the molecular ion while oxygen (O) occupies the terminals, one on each side. There are a total of 4 electron density regions around the central S atom in the Lewis structure of [SO3]2-.

SO3 Molecular Geometry / Shape and Bond Angles (Sulfur Trioxide) YouTube
The Lewis structure for SO 32- is requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SO 3 at first. Remember, Sulfur is in Period 3 and can hold more than 8 valence electrons.

So3 Lewis Structure 2 JalentuGentry
Draw the Lewis structure for the sulfite ion, SO3 2−. Which of the statements below is true for the Lewis structure of the sulfite ion? a)There are double bonds between the sulfur atom and each of the three oxygen atoms. b)There must be a double bond between the sulfur atom and one of the oxygen atoms to ensure that all atoms have an octet.

michiganswebdesigners Sif6 2 Lewis Structure
Lewis structure of SO3 The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation.
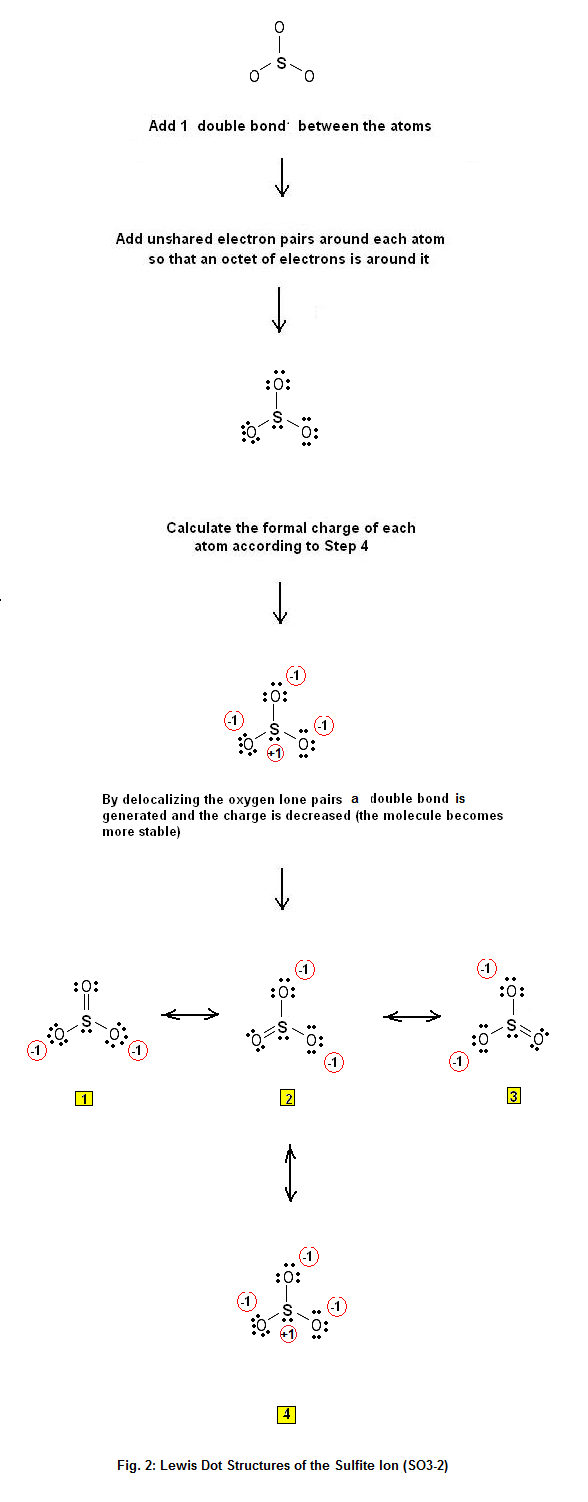
Lewis Dot Structure of the sulfite ion SO32 Electron Dot Structure Advanced Chemistry
SO3, known as sulphur trioxide is sp2 hybridized with a triagonal planar structure and having bond angle 1200. It is a colourless or white crystalline solid with boiling and melting point 450C and 16.90C respectively. It is a covalent compound having total three double bonds in between sulphur and oxygen are present in SO3 structure.

Lewis Structure of Sulfite Ion (SO3 2) YouTube
We are going to determine the Lewis structure for SO3 2- minus ion, also known as sulfide ion. The -2 charge you see is because it accepts two additional electrons, giving the ion a negative charge. Step-by-Step Guide to Drawing the SO3 2- Lewis Structure 1. Count Valence Electrons

SO3 2 Lewis Structure How to Draw the Lewis Structure for SO3 2 (Sulfite Ion) YouTube
Wayne Breslyn 724K subscribers Join Subscribe Subscribed 1.9K Share 411K views 10 years ago A step-by-step explanation of how to draw the SO3 2- Lewis Structure (Sulfite Ion). For the SO3.

SO3 Lewis Structure, Molecular Geometry, and Hybridization Techiescientist
Following steps are required to draw the SO 32- lewis structure and they are explained in detail in this tutorial. Find total number of electrons of the valance shells of sulfur and oxygen atoms Total electrons pairs Center atom selection Put lone pairs on atoms Check the stability and minimize charges on atoms by converting lone pairs to bonds.

Draw The Lewis Structure Of So3 2 Fotodtp
To sketch the SO3 Lewis structure by following these instructions: Step-1: SO3 Lewis dot Structure by counting valence electrons on the sulfur atom. Step-2: Lewis Structure of SO3 for counting valence electrons around the terminal oxygen atoms. Step-3: Lewis dot Structure for SO3 generated from step-1 and step-2.

Image Showing Resonance Strcuture Of So3 So3 Resonance Structures Clipart Full Size Clipart
Solution Verified by Toppr The molecular geometry of S O 3 2 − is a trigonal pyramidal structure with bond angles of 107.5 degrees. S O 3 2 − = Total valence electrons = 6 e + 3 × 6 e + 2 e = 26 e Formal charge on central atom = 6 − [ 2 + 1 2 × 8] = 0 Sulfur, the central atom has: 1. 1 lone pair, and 2. 3 bonds
[Solved] 1) Draw the Lewis structure of SO3 2 a) Draw the resonance... Course Hero
A quick explanation of the molecular geometry of SO3 2- (Sulfite ion) including a description of the SO3 2- bond angles.Looking at the SO3 2- Lewis structure.